
Quality
Applying quality standards in all our day-to-day activities


Applying quality standards in all our day-to-day activities

What goes into providing Quality Medical Laboratory services? At CILM, it includes generating reliable, on-time patient test reports using the appropriate technologies; meeting internal standards thanks to committed and competent staff; and carefully following all laboratory policies and procedures, including by ensuring the necessary level of documentation. In our day-to-day operations, we seek to provide a safe environment or all staff and patients.

Our activities include:
We also participate in the Annual External Quality Assessment (EQA) program for HIV Viral Load Testing, HBV Viral Load Testing, HCV Viral Load Testing, HBV Serology Testing and HIV Drug Resistant Testing.
We ensure the security of patient information management systems, samples systems, and laboratory entrance systems.
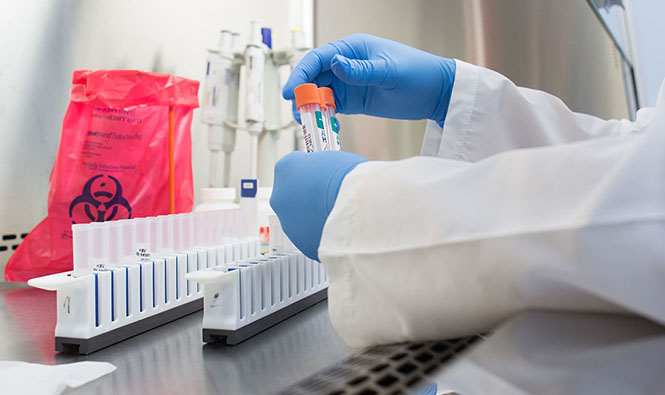
CILM received ISO15189:2012 and ISO15190:2020 accreditation for HBV Viral Load & Xpert MTB/RIF on Sputum specimen testing. Credited Number: 4224/62